Dr. Pascal Salaün from University of Liverpool is measuring on FRidge cruise inorganic and organic sulfidic compounds. Sulfide is mostly present in seawater (pH 8) as hydrogen sulfide HS– (95%) and the volatile H2S sulfide (4-5%), sulfide being very low. These sulfide are produced in reducing environments (pore waters, vents) and are not stable in oxygenated environment. They form strong complexes with several metals (Cu, Fe, Zn, Hg, Ag, etc. …). The organic sulfidic compound that can also be measured here are thiols (containing a group –SH). They are more stable than sulphide species and are thought to be produced by cells as anti-oxidant and/or in response to metal stress but they are present at very low concentrations (low nM levels).

To measure these components, Dr. Pascal Salaün is using the method of Cathodic Stripping Voltammetry. This electroanalytical method is based on a time dependent potential that is applied to a flow cell containing three electrodes: a working electrode in Mercury (Hg-Au amalgam formed by the deposition of a mercury film on a gold wire), an auxiliary electrode (carbon) and a reference electrode. The resulting current is then measured as a function of that potential.
The Voltammeter (on the left) and a zoom on the flow cell in the faraday cage (on the right) with the 3 electrodes. © Lise Artigue
This method proceeds along the following steps:
- Cleaning/reset step: A low potential is applied to remove any sulphide from the electrode.
- Deposition/Precipitation: The mercury is oxidised by the sulfide forming a precipitate HgS that accumulates at the surface of the electrode (HS– + Hg -> HgS + H+ + 2 e–).
- Stripping: The mercury from the newly formed precipitate is reduced during a cathodic potential sweep (HgS + H+ + 2 e– -> HS– + Hg) giving off electrons which are measured as a current. The intensity of the current is directly proportional to the concentration.
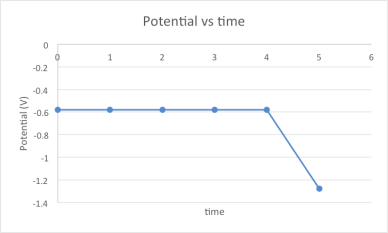
Figure showing the potential applied during the accumulation and stripping steps.
To distinguish inorganic and organic sulfidic compounds measurements, the deposition potential can be varied. The advantage of this method is a low detection limit, a good reproducibility, and samples are analysed as they are (non-buffered and oxygenated). Moreover, the flow cell is easy to change, and avoid the votalisation of H2S.
The difficulties of this sulfidic compounds measurements is the short half-life of sulfide (22h for H2S just with O2) which impose to be measured quickly after the sampling. Measurements are done as soon as the rosette comes up and concentrations can be compared with other unstable analytes (FeII, MnII) that are also measured on-board.
During this cruise, Dr. Pascal Salaün is hoping to “measure sulphide in the proximity of the vents and relate these levels to FeII, MnII and other chemical indicators to get a better understanding of the transport of these short-lived species along the plume”.
Lise Artigue

